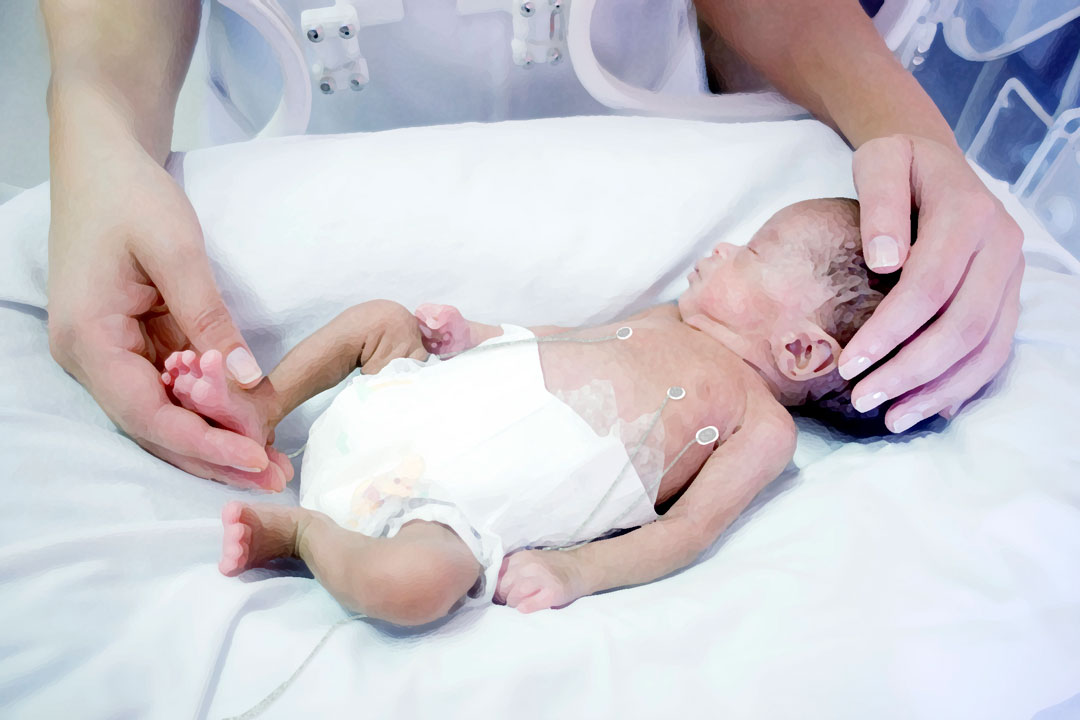
Caring for our most vulnerable patients
Revolutionizing neonatal care
Every year, approximately 15 million children worldwide – about one in ten – are born preterm (prior to pregnancy week 37). Significant progress has been made to increase the survival rate of preterm born infants over the past 50 years, however, approximately 1.1 million infants still die every year.
The most common cause of death for preterm born infants is respiratory distress syndrome (RDS), a serious lung condition whose complications can affect up to 80 percent of infants born extremely preterm. The methods used by neonatal health care professionals today involve manual observation with help from e.g. chest x-rays and blood samples. These methods do not provide continuous information for decision making. They can also cause serious discomfort and pain for patients, a higher risk for developing cancer later on in life and high health care costs.
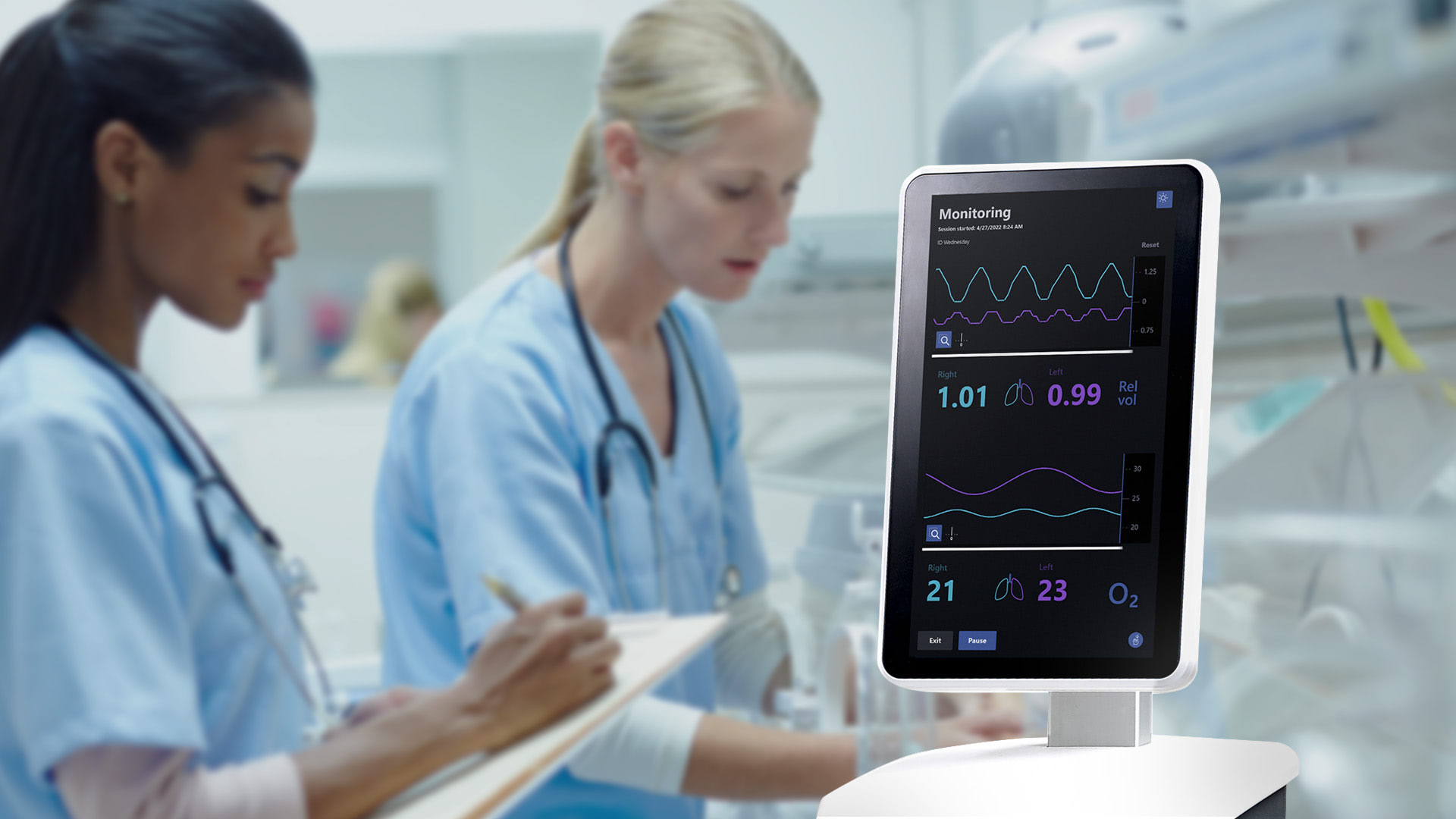
Neola® – continuous, safe and noninvasive lung monitoring for preterm born infants
Neola Medical’s Neola® can offer a safe and non-invasive way to continuously measure lung volume changes and oxygen gas saturation in the lungs of preterm born infants with the possibility of instantly detecting complications such as an obstructed airway or a misplaced tracheal tube. This means that health care personnel are alerted to problems in real time and can treat patients right away.
Neola® uses a biomedical application of GASMAS* technology originally developed by researchers at the Division of Atomic Physics at Lund University. Small and lightweight emitter and detector probes are positioned on the skin. Near-infrared light is sent through the chest cavity, where a small portion is absorbed by water vapor and oxygen gas molecules in the lungs. The detector probe detects some of the light that travelled through the tissue and the signal shows how much of the light that has been absorbed by the gas molecules. Using this data, Neola® can register small changes related to lung volume and oxygen gas concentration. The probes can be easily removed and repositioned, avoiding discomfort and stress for the infant.
A gamechanger in the pediatric landscape
Development of medical technology has almost exclusively focused on devices for adults. The FDA has acknowledged this discrepancy and highlighted the need for more pediatric medical devices. The FDA has therefore taken concrete steps to incentivize development of medical devices for children and infants.
Several factors suggest that Neola Medical (publ) with Neola® has the potential to evolve and upgrade the care of preterm born infants: increased regulatory focus on developing pediatric medical devices, the United Nations Global Agenda 2030 partial goal of reduced neonatal mortality caused by preventable conditions (Goal 3.2), the positive investment climate of the medical technology sector as well as a historic Swedish tradition of leading the way in innovations related to medical technology and neonatal care.
*GAs in Scattering Media Absorption Spectroscopy (GASMAS)
Emilie Krite Svanberg, MD Ph.D., and Dennis Leander, MSc, Neola Medical, during clinical pilot study on healthy newborn infants at Skåne University Hospital in the fall of 2018.
For Investors
We are on a journey to revolutionize neonatal intensive care
Learn more about investing in Neola Medical